Is Cu(iii) a necessary intermediate in Cu-mediated coupling reactions? A mechanistic point of view
Abstract
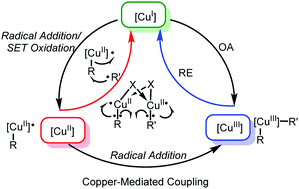
The mechanism of copper-mediated coupling reactions has aroused widespread interest, and it has been found to not be as simple as initially thought. In this article, we give an overview of the recent advances in this field. Notably, we focus on whether the presence of CuIII is adopted in the catalytic cycle. Attention is paid to the key CuII species, which can be generated by radical-type or single electron transfer (SET) oxidation of CuI. The CuII species can be oxidized to CuIII by SET or using a nucleophilic radical for further reductive elimination. Alternatively, radical-type substitution or bimetallic reductive elimination can avoid formation of CuIII, and coupling reactions can also be achieved.


 Please wait while we load your content...
Please wait while we load your content...