N-Terminal speciation for native chemical ligation†
Abstract
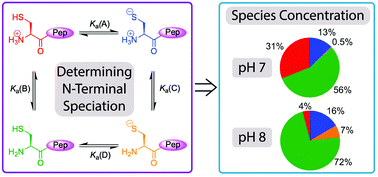
Native chemical ligation (NCL) enables the chemical synthesis of peptides via reactions between N-terminal thiolates and C-terminal thioesters under mild, aqueous conditions at pH 7–8. Here we demonstrate quantitatively how thiol speciation at N-terminal cysteines and analogues varies significantly depending upon structure at typical pH values used in NCL.


 Please wait while we load your content...
Please wait while we load your content...