Uncovering the pathological functions of Ser404 phosphorylation by semisynthesis of a phosphorylated TDP-43 prion-like domain†
Abstract
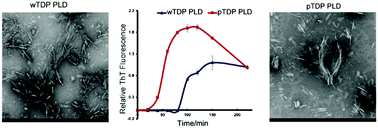
Herein, we have successfully semi-synthesized a TDP-43 prion-like domain with Ser404 phosphorylation. We have demonstrated that Ser404 phosphorylation could accelerate the amyloid aggregation of the TDP-43 prion-like domain and aggravate its cytotoxicity.


 Please wait while we load your content...
Please wait while we load your content...