Synthesis of chiral α-substituted α-amino acid and amine derivatives through Ni-catalyzed asymmetric hydrogenation†
Abstract
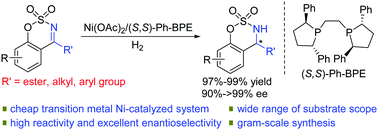
Highly efficient Ni-catalyzed asymmetric hydrogenation of cyclic N-sulfonyl ketimino esters was, for the first time, successfully developed, providing various chiral α-monosubstituted α-amino acid derivatives with excellent results (97–99% yields, 90 to >99% ee). Cyclic N-sulfonyl ketimines were also hydrogenated well to afford chiral amine derivatives with 98–99% yields and 97 to >99% ee. The gram-scale asymmetric hydrogenation was performed well with 85% yield and 99% ee using only 0.2 mol% catalyst.


 Please wait while we load your content...
Please wait while we load your content...