Assembly of polysubstituted chiral cyclopropylamines via highly enantioselective Cu-catalyzed three-component cyclopropene alkenylamination†
Abstract
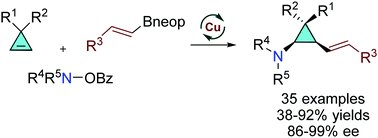
Enabled by a commercial bisphosphine ligand, the Cu-catalyzed three-component cyclopropene alkenylamination with alkenyl organoboron reagent and hyroxyamine esters proceeds with exceptionally high enantioselectivity to deliver poly-substituted cis-1,2-alkenylcyclopropylamines that contain up to all three stereogenic centers on the cyclopropane.


 Please wait while we load your content...
Please wait while we load your content...