Conformational control of N-methyl-N,N′-diacylhydrazines by noncovalent carbon bonding in solution†
Abstract
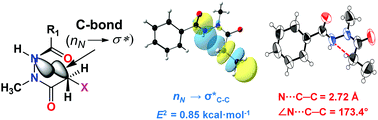
In recent years, some X-ray structural and computational evidence has emerged for noncovalent carbon bonding (C-bond). However, evidence of C-bonds in solution is limited. Herein, from the conformational analyses of strategically designed N-methyl-N,N′-diacylhydrazines, we for the first time show that C-bonds can be modulated to control the conformational preferences of small molecules in solution. We show that unusual N(amide)⋯C–X noncovalent carbon bonding interactions stabilize the trans–cis (t–c) amide bond rotamers of N-methyl-N,N′-diacylhydrazines over the expected trans–trans (t–t) rotamers.


 Please wait while we load your content...
Please wait while we load your content...