Visualization of crystal plane selectivity for irreversible phase transition in MnO@C anode†
Abstract
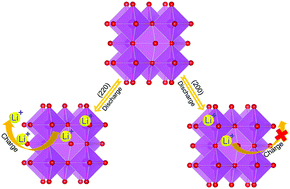
Transition metal oxides are widely regarded as one of the most promising candidates for lithium-ion battery (LIB) anodes. However, the mechanisms of irreversible reactions occurring during the charging/discharging process are still controversial. In this study, the atomic structural transitions of the MnO@C anode upon lithiation/delithiation at the first cycle of charging and discharging are elucidated. Based on the quantities of Li embedded and released in different states, the anisotropy of the crystal plane of lithiation/delithiation in MnO is directly observed. We determine that lithium ions can be completely inserted into/extracted from MnO(220), while this cannot be achieved in MnO(200), which is the main reason for capacity degradation. This study reveals the reaction mechanisms and structural evolution in the electrochemical reactions of MnO@C anode materials during lithiation and delithiation. Additionally, it also provides guidance for the fabrication and optimization of MnO-based materials for LIBs in the future.


 Please wait while we load your content...
Please wait while we load your content...