Modular synthesis of α-aryl β-perfluoroalkyl ketones via N-heterocyclic carbene catalysis†
Abstract
A new general de novo synthesis of pharmaceutically important α-aryl β-perfluoroalkyl ketones has been disclosed. Compared with trifluoromethylation-initiated radical 1,2-aryl migration of α,α-diaryl allylic alcohols, this protocol employs a new strategy of biomimetic carbene catalysis to assemble alkene, aldehyde and perfluoroalkyl reagents, providing access to products with excellent flexibility of the aryl unit and perfluoroalkyl group. This method also demonstrates excellent functional group compatibility, including some Grignard reagent sensitive groups.
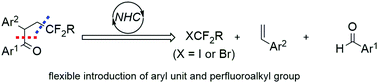


 Please wait while we load your content...
Please wait while we load your content...