Catalyst and additive-free oxidative dual C–H sulfenylation of imidazoheterocycles with elemental sulfur using DMSO as a solvent and an oxidant†
Abstract
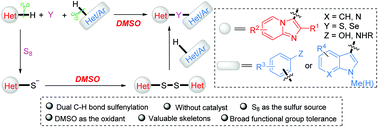
Dual C–H sulfenylation was used to obtain 3-vulcanized imidazoheterocycles using odorless elemental sulfur under catalyst- and additive-free conditions. C–H activation of both imidazoheterocycles and arylamines/arenols/indoles was realized by a practical protocol in which DMSO served as both a solvent and an internal oxidant.
- This article is part of the themed collection: Editor’s Choice: Main group reagents and catalysts in organic reactions


 Please wait while we load your content...
Please wait while we load your content...