Peroxidation of 3,4-dihydro-1,4-benzoxazin-2-ones†
Abstract
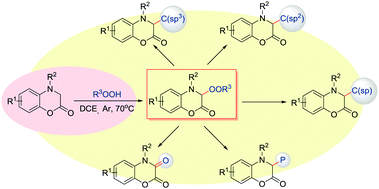
The sp3-C–H peroxidation of 3,4-dihydro-1,4-benzoxazin-2-ones was achieved under mild and simple catalyst-free reaction conditions. A range of biologically important alkylated benzoxazinone peroxides are synthesized in high yield with a good functional group tolerance. The C(sp3)-OO bond was constructed efficiently and could be further converted into C(sp3)–C(sp3), C(sp3)–C(sp2), C(sp3)–C(sp), C–P and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds by late-stage functional group transformations.
O bonds by late-stage functional group transformations.


 Please wait while we load your content...
Please wait while we load your content...