Rhodium-catalyzed annulative coupling of N-aryl-2-aminopyridine and propargylic amine via selective C–C and C–H bond activation†
Abstract
A Rh(III)-catalyzed/Cu(II)-mediated cascade reaction between N-aryl-2-aminopyridine and propargylic amine has been developed. Selective C(sp2)–H bond activation and C(sp)–C(sp3) cleavage occurred during the reaction, which was followed by a cyclization reaction to provide an unprecedented synthetic route to form 1,2-disubstituted indoles in yields up to 85%.
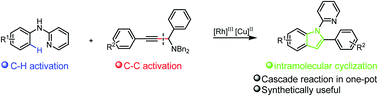


 Please wait while we load your content...
Please wait while we load your content...