Irreversible tautomerization as a powerful tool to access unprecedented functional porous organic polymers with a tris(β-keto-hydrazo)cyclohexane subunit (TKH-POPs)†
Abstract
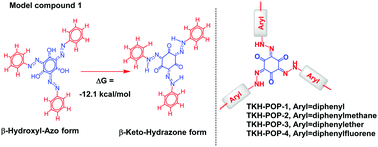
Porous organic polymers (POPs) have received much attention, due to their multiple potential applications and flexibility in chemical structure design. Creation of a novel chemical structure has been the central task in the research of POPs, which are usually constructed by direct coupling polymerizations. The fascinating rearrangement/tautomerization could lead to some novel structures, which are hard to access by conventional direct coupling polymerizations. Herein, the tautomerization from tris(β-hydroxyl-azo)benzene to the tris(β-keto-hydrozo)cyclohexane structure has been proved unambiguously based on an advanced 2D NMR technique such as 15N–1H-HSQC and 1H–1H-NOESY. The crucial tautomerization was used to synthesize TKH-POPs for the first time. The as-synthesized TKH-POP-1 was found to have an adsorption capacity as high as 66.3 mmol g−1 (at 273 K and P/P0 = 0.98) towards acetonitrile vapor, which was the highest among all the reported materials. The general and flexible strategy to make functional POPs with tunable pores such as ultramicropores, micropores and mesopores will help develop interesting functional POPs in the near future.
- This article is part of the themed collection: Chemical Communications HOT Articles


 Please wait while we load your content...
Please wait while we load your content...