A carboxylate-assisted amination/unactivated C(sp2)–H arylation reaction via a palladium/norbornene cooperative catalysis†
Abstract
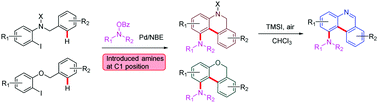
This report describes a carboxylate-assisted palladium-catalysed Catellani reaction, which is compatible with ortho-amination and unactivated C(sp2)–H arylation. This method was used to synthesize a series of 1-amino substituted dihydrophenanthridines, phenanthridines and 6H-benzo[c]chromenes. Based on kinetic isotope experiments, the kinetic curve proves that pivalic acid accelerates the reaction rate of unactivated C(sp2)–H activation, and thus this rate can keep up with the five membered aryl-norbornene–palladacycle (ANP) intermediate.


 Please wait while we load your content...
Please wait while we load your content...