Borohydride intermediates pave the way for magnesium-catalysed enantioselective ketone reduction†
Abstract
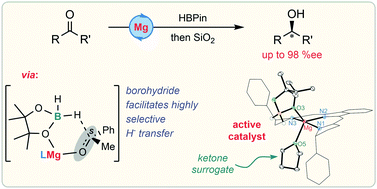
A magnesium precatalyst for the highly enantioselective hydro-boration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds is reported. The mechanistic basis of the unprecedented selectivity of this transformation has been investi-gated experimentally by isolation of catalytic intermediates and theoretically by DFT calculations. The facile formation of a magnesium borohydride species is critical in overcoming competing pathways in the selectivity-determining insertion step.
O bonds is reported. The mechanistic basis of the unprecedented selectivity of this transformation has been investi-gated experimentally by isolation of catalytic intermediates and theoretically by DFT calculations. The facile formation of a magnesium borohydride species is critical in overcoming competing pathways in the selectivity-determining insertion step.
- This article is part of the themed collection: Chemical Communications HOT Articles


 Please wait while we load your content...
Please wait while we load your content...