Disulfiram as a potent metallo-β-lactamase inhibitor with dual functional mechanisms†
Abstract
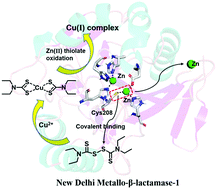
We report a promising NDM-1 inhibitor, disulfiram, which can covalently bind to NDM-1 by forming an S–S bond with the Cys208 residue. Its copper-containing metabolite in vivo, Cu(DTC)2, also inactivated NDM-1 through oxidizing the Zn(II) thiolate site of the enzyme, therefore exhibiting dual functional inhibitory potential against B1 and B2 subclass MβLs.


 Please wait while we load your content...
Please wait while we load your content...