Frustrated Lewis pair-catalyzed double hydroarylation of alkynes with N-substituted pyrroles†
Abstract
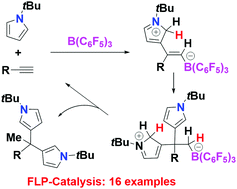
Metal-free hydroarylation of alkynes with N-substituted pyrroles is shown to be most efficiently mediated by B(C6F5)3 to yield 12 variants of dipyrrole-alkanes, a mono-hydroarylation product and a tetrahydroarylation product of a bis-alkyne. These products were generally obtained in good to excellent yields (up to 95%). Control experiments suggest a mechanism involving FLP addition of the borane and pyrrole to alkyne.
- This article is part of the themed collection: Editor’s Choice: Main group reagents and catalysts in organic reactions


 Please wait while we load your content...
Please wait while we load your content...