Light-responsive vesicles for enantioselective release of chiral drugs prepared from a supra-amphiphilic M-helix†
Abstract
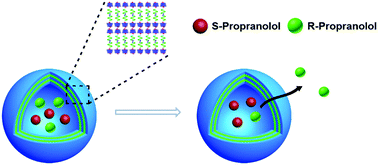
A kind of light-responsive vesicle was prepared by aqueous self-assembly of α-CD and an azobenzene-containing M-helical foldamer, which displayed dynamic disassembly–reassembly structural transformation when alternately irradiated by UV and visible light. Distinctively, this vesicle also exhibited enantioselective release abilities toward racemic propranolol (a β-blocker), owing to the M-helical building blocks.


 Please wait while we load your content...
Please wait while we load your content...