Abstract
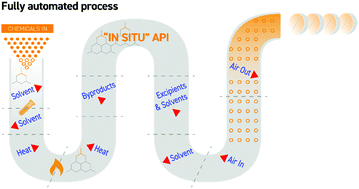
We report here a fully automated, end-to-end, integrated continuous manufacturing process for a small-molecule generic medication with built-in quality assurance. The entire process fits into a box of 30.7 m2 modular footprint and a total residence time of less than 30 h, with a throughput up to 40.3 × 106 tablets per year.


 Please wait while we load your content...
Please wait while we load your content...