Synthesis of planar chiral ferrocenes via a Pd(0)-catalyzed syn-carbopalladation/asymmetric C–H alkenylation process†
Abstract
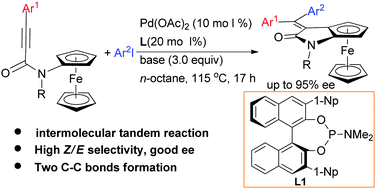
The Pd(0)-catalyzed tandem intermolecular syn-carbopalladation/asymmetric C–H alkenylation reaction of N-ferrocenyl propiolamides with aryl iodides has been realized, generating planar chiral ferrocene[1,2-d] pyrrolinones in good yields. Through employing BINOL-derived phosphoramidite ligands, up to 95% ee is achieved.


 Please wait while we load your content...
Please wait while we load your content...