Completely regioselective insertion of unsymmetrical alkynes into electron-deficient alkenes for the synthesis of new pentacyclic indoles†
Abstract
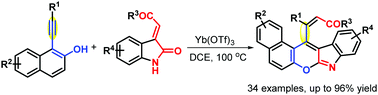
A Lewis acid-catalyzed insertion of unsymmetrical alkynes into electron-deficient alkenes was developed for the first time, and used to produce 34 hitherto unreported pentacyclic benzo[5,6]chromeno[2,3-b]indoles with generally good yields and complete stereoselectivity. A Yb(OTf)3-catalyzed reaction between o-alkynylnaphthols and 3-methyleneindolin-2-ones proceeded efficiently, and provided a simple and convergent protocol for alkyne difunctionalization via oxidant-free C–C double bond breaking/rearrangement. Mechanistic details of this domino process were derived by conducting systematic theoretical calculations.


 Please wait while we load your content...
Please wait while we load your content...