Silver-promoted cascade radical cyclization of γ,δ-unsaturated oxime esters with P(O)H compounds: synthesis of phosphorylated pyrrolines†
Abstract
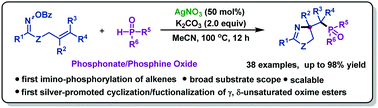
A cascade radical cyclization was realized for the first silver-promoted imino-phosphorylation of γ,δ-unsaturated oxime esters, which provided a step-economical and redox-neutral route to access a variety of phosphorylated pyrrolines in good to excellent yields. Moreover, a new bulky trivalent phosphine ligand with a pyrroline motif was obtained through a deoxidation process.


 Please wait while we load your content...
Please wait while we load your content...