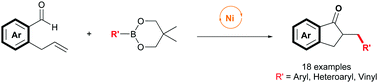
Nickel-catalyzed exo-selective hydroacylation/Suzuki cross-coupling reaction†
Abstract
The first nickel-catalyzed intramolecular hydroacylation/Suzuki cross coupling cascade of o-allylbenzaldehydes with a broad range of phenylboronic acid neopentyl glycol esters has been developed. This strategy shows high regioselectivity and step economy in the construction of two C–C bonds via aldehyde C–H bond activation, affording valuable indanones with high efficiency.


 Please wait while we load your content...
Please wait while we load your content...