A novel distyryl boron dipyrromethene with two functional tags for site-specific bioorthogonal photosensitisation towards targeted photodynamic therapy†
Abstract
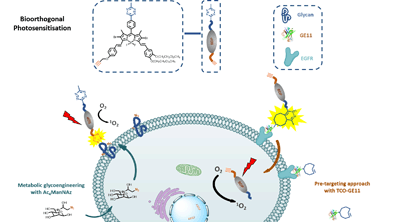
A distyryl boron dipyrromethene based photosensitiser substituted with 1,2,4,5-tetrazine and alkyne moieties was prepared. Through site-specific bioorthogonal reactions with the complementary functional tags anchored on the membrane of A431 human epidermoid carcinoma cells, this versatile photosensitiser exhibited enhanced cellular uptake and photocytotoxicity. The bioorthogonal ligation was also demonstrated in tumour-bearing nude mice.


 Please wait while we load your content...
Please wait while we load your content...