Nucleophilic halo-Michael addition under Lewis-base activation†
Abstract
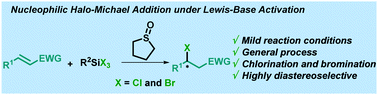
A simple and general conjugate nucleophilic halogenation is presented. The THTO/halosilane combination has shown the ability to act as a nucleophilic halide source in the conjugate addition to a variety of Michael acceptors. In addition, a straightforward diastereoselective halogen installation using α,β-unsaturated acyloxazolidinones as platforms has been developed.


 Please wait while we load your content...
Please wait while we load your content...