Enantioselective palladium-catalyzed diarylation of unactivated alkenes†
Abstract
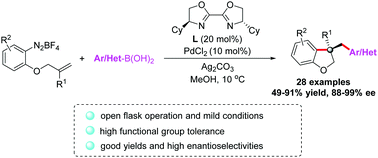
Enantioselective Pd-catalyzed diarylation of unactivated alkenes between arenediazonium salts and arylboronic acids has been developed. This method provides an efficient route to dihydrobenzofurans with all-carbon quaternary centers in good yields with 88–99% ee. This reaction proceeding under mild and open-flask conditions is compatible with a variety of functional groups, including cyano, ketone, ester, amide, bromine and free hydroxyl groups.


 Please wait while we load your content...
Please wait while we load your content...