Palladium/copper-catalyzed multicomponent reactions of propargylic amides, halohydrocarbons and CO2 toward functionalized oxazolidine-2,4-diones†
Abstract
A palladium/copper-catalyzed oxy-carbonation of propargylic amides by halohydrocarbons and CO2 has been developed toward functionalized oxazolidine-2,4-diones. This multi-component reaction (MCR) was triggered by the oxidative addition of RX to Pd(0), followed by the sequential carboxylation of amide and trans-oxopalladation of an electron-deficient triple bond by RPdX species. Finally, the reductive elimination afforded products possessing tetra-substituted vinyl motifs and Pd(0). This protocol features simultaneous formation of three bonds, representing an efficient method for incorporation of CO2 into value-added heterocycles.
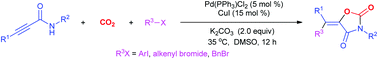


 Please wait while we load your content...
Please wait while we load your content...