Thiyl radical promoted iron-catalyzed-selective oxidation of benzylic sp3 C–H bonds with molecular oxygen†
Abstract
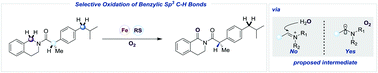
A ligand-free iron-catalyzed method for the oxygenation of benzylic sp3 C–H bonds by molecular oxygen (1 atm) using a thiyl radical as a cocatalyst has been developed. This transformation provides a facile access to amides, esters and ketones from readily accessible corresponding amines, ethers and alkanes. It features high regioselectivity, mild oxidative conditions and excellent functional group compatibility, providing good opportunities to the site-selective functionalization of complex molecules. Preliminary mechanistic studies suggest that this reaction may not undergo a benzylic cation intermediate pathway and the carbonyl oxygen atom in the products may be derived from molecular oxygen.


 Please wait while we load your content...
Please wait while we load your content...