Self-sorting in dynamic disulfide assembly: new biphenyl-bridged “nanohoops” and unsymmetrical cyclophanes†
Abstract
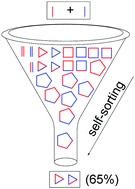
We expand on our approach combining dynamic covalent self-assembly and sulfur extrusion to synthesize new biphenyl-linked disulfide and thioether macrocycles, which are variants of the venerable phenyl-bridged paracyclophanes. We then advance this strategy further to use two different thiols in tandem to provide new, elusive unsymmetrical disulfides which can also be trapped as unsymmetrical thioether “nanohoops”. This approach enables substantial amplification of two unsymmetrical trimers out of a library of at least 21 possible macrocycles of various sizes.


 Please wait while we load your content...
Please wait while we load your content...
