Highly isoselective ring-opening polymerization of rac-O-carboxyanhydrides using a zinc alkoxide initiator†
Abstract
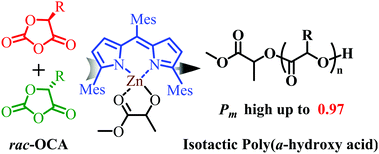
The stereoselective ring-opening polymerization (ROP) of O-carboxyanhydrides (OCAs) remains a major challenge due to the easy epimerization of monomers. In this work, using a zinc alkoxide initiator, highly efficient ROP of enantiopure 5-methyl-1,3-dioxolane-2,4-dione (LacOCA), 5-benzyl-1,3-dioxolane-2,4-dione (PheOCA), and 5-(4-(benzyloxy)benzyl)-1,3-dioxolane-2,4-dione (Try(Bn)OCA) was achieved without obvious epimerization. Moreover, highly isoselective ROP of rac-LacOCA, rac-PheOCA, and rac-Try(Bn)OCA was successful with the highest isoselectivity of Pm = 0.97 at −70 °C.


 Please wait while we load your content...
Please wait while we load your content...