Anion amphiprotic ionic liquids as protic electrolyte matrices allowing sodium metal plating†
Abstract
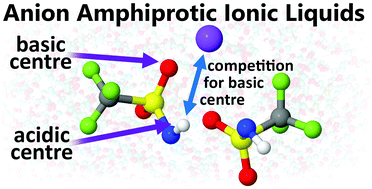
The sodium-ion battery (SIB) is proposed as a complementary technology to today's commercially dominant lithium-ion battery (LIB). While much know-how can be transferred from LIBs to SIBs, adjustments are still necessary, not the least for the electrolytes employed. Here the use of anion amphiprotic ionic liquid (AAIL) based electrolytes is proposed for SIB application. Two different AAILs, based on organic trifluoromethylsulfonylamide (TFSAm) and inorganic HSO4− anions, respectively, doped with NaTFSI salt have been studied, focusing on electrochemical stability and transport properties, complemented by studies of the ion–ion interactions, and final sodium-ion battery performance via stripping/plating vs. sodium metal electrodes.


 Please wait while we load your content...
Please wait while we load your content...