A promising water-in-salt electrolyte for aqueous based electrochemical energy storage cells with a wide potential window: highly concentrated HCOOK
Abstract
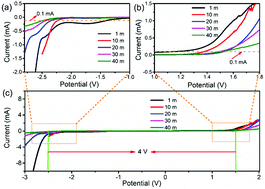
Recently, water-in-salt electrolytes have been widely reported because of their ability in broadening the potential window of aqueous based energy storage devices. Herein, another eco-friendly and cost-effective electrolyte, concentrated potassium formate of 40 M HCOOK where the water-to-salt molar ratio falls to 1.38 : 1, is proposed. The electrolyte demonstrates a wide potential window of up to 4 V (−2.5 to 1.5 V vs. Ag/AgCl) with a glassy carbon electrode. Compared with a CH3COOK based electrolyte, the HCOOK possesses lower stable negative potential and higher ionic conductivity. For an activated carbon based supecapacitive electrode, a low discharge potential of −2.4 V vs. Ag/AgCl can be achieved. Besides, a high capacity of 321 F g−1 is obtained at 5 A g−1 and it is still as high as 121 F g−1 at 20 A g−1. Meanwhile, typical K-battery behavior is exhibited for the KTi2(PO4)3 anode and a reversible capacity of 15 mA h g−1 can be delivered at 0.1 A g−1.


 Please wait while we load your content...
Please wait while we load your content...