Intercepted dehomologation of aldoses by N-heterocyclic carbene catalysis – a novel transformation in carbohydrate chemistry†
Abstract
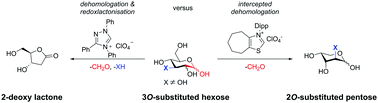
The development of an N-heterocyclic carbene (NHC) catalysed intercepted dehomologation of aldoses is reported. The unique selectivity of NHCs for aldehydes is exploited in the complex context of reducing sugars. Examples of strong substrate governance for either intercepted dehomologation or a subsequent redox-lactonisation were identified and mechanistically understood. More importantly, it was shown that catalyst design allowed the tuning of the selectivity of the reaction with structurally unbiased starting materials towards either of the two scenarios.


 Please wait while we load your content...
Please wait while we load your content...