Dioxazolones as masked ester surrogates in the Pd(ii)-catalyzed direct C–H arylation of 6,5-fused heterocycles†
Abstract
A simple and effective Pd(II)-catalyzed regioselective C(2)–H arylation of 6,5-fused heterocycles with dioxazolones as a masked ester surrogate under mild conditions is reported. The significance of the arylation is highlighted by the new reactivity demonstrated in dioxazolones via proximal C–H activation of the cyclic carbonate of the hydroxamic acid functionality under protic conditions.
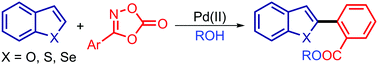


 Please wait while we load your content...
Please wait while we load your content...