Trisulfides over disulfides: highly selective synthetic strategies, anti-proliferative activities and sustained H2S release profiles†
Abstract
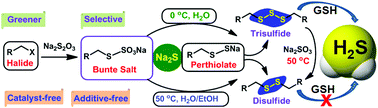
Temperature- and solvent-induced selective synthesis of trisulfides and disulfides is demonstrated. A remarkable selectivity was achieved using Na2S as a sulfur-transfer agent under mild, greener, catalyst-free and additive-free conditions. This study reveals trisulfides as a better model than disulfides in general for a sustained release of H2S and potent anti-cancer activities.


 Please wait while we load your content...
Please wait while we load your content...