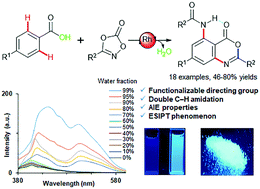
Rhodium(iii)-catalyzed cascade reactions of benzoic acids with dioxazolones: discovery of 2,5-substituted benzoxazinones as AIE molecules†
Abstract
A rhodium-catalyzed cascade reaction of benzoic acids with 1,4,2-dioxazol-5-ones was studied. The carboxyl group enabled a double C–H amidation followed by further intramolecular cyclization to afford 2,5-substituted benzoxazinones, which exhibited aggregation-induced emission (AIE) properties with a promising excited-state intramolecular proton-transfer (ESIPT) phenomenon.


 Please wait while we load your content...
Please wait while we load your content...