Mechanistic insights into the gold(i)-catalyzed annulation of propiolates with isoxazoles: a DFT study†
Abstract
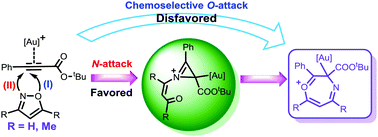
The detailed mechanisms of gold(I)-catalyzed annulations of propiolates with substituted and unsubstituted isoxazoles were investigated by DFT calculations. A unified rationale for the formation of the key seven-membered heterocyclic intermediate was proposed through initial chemoselective N-attack of isoxazole followed by sequential generation of an unprecedented 2H-azirine-containing intermediate, 6π electrocyclization and ring expansion. Subsequent substrate-dependent transformations were rationalized to generate the divergent products. The origins of the chemo- and regio-selectivities coupled with the factors responsible were addressed.
- This article is part of the themed collection: SU 120: Celebrating 120 Years of Soochow University


 Please wait while we load your content...
Please wait while we load your content...