Macrocyclisation and functionalisation of unprotected peptides via divinyltriazine cysteine stapling†
Abstract
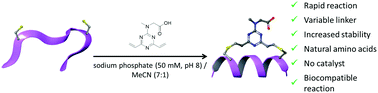
We report a novel divinyltriazine linker for the stapling of two cysteine residues to form macrocyclic peptides from their unprotected linear counterparts. The stapling reaction occurred rapidly under mild conditions on a range of unprotected peptide sequences. The resulting constrained peptides displayed greater stability in a serum stability assay when compared to their linear counterparts.


 Please wait while we load your content...
Please wait while we load your content...