Promoting water dissociation performance by borinic acid for the strong-acid/base-free hydrogen evolution reaction†
Abstract
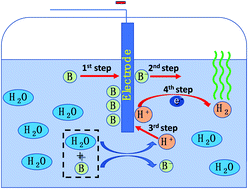
A new type of organic proton donor for the strong-acid/base-free hydrogen evolution reaction is reported, by taking advantage of the electron deficiency of the boron center of borinic acid and the strong affinity and hydrolysis reaction between borinic acid and water with hydrogen ion formation. The borinic acid proton donor exhibits even higher current density than H2SO4 to promote hydrogen evolution performance in DMF.


 Please wait while we load your content...
Please wait while we load your content...