The thermally induced decarboxylation mechanism of a mixed-oxidation state carboxylate-based iron metal–organic framework†
Abstract
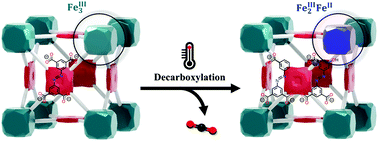
Investigations into a thermally generated decarboxylation mechanism for metal site activation and the generation of mesopores in a carboxylate iron-based MOF, PCN-250, have been conducted. PCN-250 exhibits an interesting oxidation state change during thermal treatment under inert atmospheres or vacuum conditions, transitioning from an Fe(III)3 cluster to a Fe(II)Fe(III)2 cluster. To probe this redox event and discern a mechanism of activation, a combination of thermogravimetric analysis, gas sorption, scanning electron microscopy, 57Fe Mössbauer spectroscopy, gas chromatography-mass spectrometry, and X-ray diffraction studies were conducted. The results suggest that the iron-site activation occurs due to ligand decarboxylation above 200 °C. This is also consistent with the generation of a missing cluster mesoporous defect in the framework. The resulting mesoporous PCN-250 maintains high thermal stability, preserving crystallinity after multiple consecutive high-temperature regeneration cycles. Additionally, the thermally reduced PCN-250 shows improvements in the total uptake capacity of methane and CO2.


 Please wait while we load your content...
Please wait while we load your content...
