Guanidine cyclic diimides and their polymers†
Abstract
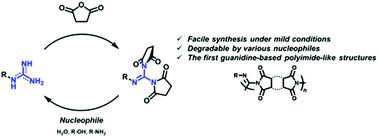
We report the formation and degradation of a unique guanidine cyclic diimide (GCDI) structure and GCDI-based polymers. The GCDI structure is readily formed under mild conditions. The X-ray crystal structure showed that the delocalized π-orbitals in the guanidine plane are significantly disrupted in the GCDI structure. Unlike amine-based imides, the GCDI structure readily degrades into the initial guanidine in protic solvents at ambient temperatures. Furthermore, poly(GCDI)s, a new category of polymers with the GCDI backbones, can be synthesized from guanidines and dianhydrides. Similar to the monomeric GCDIs, poly(GCDI)s are degraded in protic solvents unlike polyimides with high chemical stability.


 Please wait while we load your content...
Please wait while we load your content...