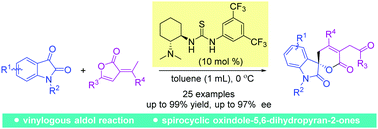
Highly enantioselective sequential vinylogous aldol reaction/transesterification of methyl-substituted olefinic butyrolactones with isatins for the construction of chiral spirocyclic oxindole-dihydropyranones†
Abstract
A highly stereoselective sequential vinylogous aldol reaction/transesterification of methyl-substituted olefinic butyrolactones and isatins was developed. With this developed protocol, a series of chiral spirocyclic oxindole-dihydropyranones were obtained in good to excellent yields (73–99%) and enantioselectivities (71–97% ee).


 Please wait while we load your content...
Please wait while we load your content...