Asymmetric synthesis of α-chiral β-hydroxy allenes: copper-catalyzed γ-selective borylative coupling of vinyl arenes and propargyl phosphates†
Abstract
Copper-catalyzed enantioselective coupling of vinyl arenes with bis(pinacolato)diboron (B2pin2) and propargylic phosphates is presented. The protocol affords a facile route to enantioenriched α-branched allenes with a versatile pinacolboronate group at the β-position with high enantioselectivity up to 98 : 2 er. In the presence of a copper catalyst complexed with a chiral bisphosphine ligand, catalytic assembly of α-chiral β-hydroxy allenes was accomplished through highly selective γ-addition of a borylalkyl copper species to propargyl substrates, followed by oxidation. Catalyst-controlled divergent cyclization reactions of the resulting allenes led to concise syntheses of enantioenriched dihydropyran and dihydrofuran rings without any loss of chiral purity.
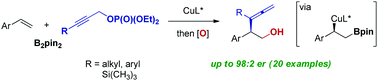


 Please wait while we load your content...
Please wait while we load your content...