Enantioselective synthesis of isoquinoline-1,3(2H,4H)-dione derivatives via a chiral phosphoric acid catalyzed aza-Friedel–Crafts reaction†
Abstract
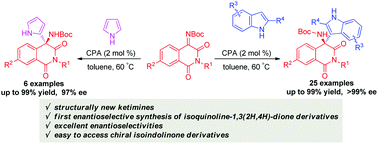
A highly enantioselective aza-Friedel–Crafts reaction of structurally new ketimines with indoles and pyrrole is developed by using a chiral phosphoric acid as the catalyst. This protocol enables the first enantioselective synthesis of isoquinoline-1,3(2H,4H)-dione derivatives in good to excellent yields (up to 99% yield) and excellent enantioselectivities (up to >99% ee).


 Please wait while we load your content...
Please wait while we load your content...