Cu-Catalyzed highly selective reductive functionalization of 1,3-diene using H2O as a stoichiometric hydrogen atom donor†
Abstract
A copper-catalyzed highly regio- and diastereo-selective reductive reaction of terminal 1,3-diene with water and aldehyde has been developed. This chemistry afforded a product containing a terminal alkenyl group, which is a versatile kind of precursor for organic synthesis, with the scope for various substrates. The present reaction system could realize the catalytic transfer of hydrogen to diene using water as a stoichiometric H atom donor. In this transformation, B2Pin2, a mild and practical kind of reductant was used as the mediator. The reaction pathway of this practical strategy was illustrated by a control experiment.
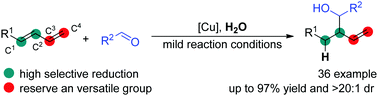
- This article is part of the themed collection: Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...