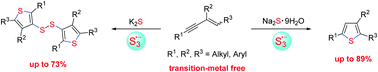
A trisulfur radical anion (S3˙−) involved sulfur insertion reaction of 1,3-enynes: sulfide sources control chemoselective synthesis of 2,3,5-trisubstituted thiophenes and 3-thienyl disulfides†
Abstract
Cascade cyclization reactions of S3˙−in situ generated from S2− with 1,3-enynes for the chemoselective synthesis of 2,3,5-trisubstituted thiophenes and 3-thienyl disulfides controlled by sulfide salts are developed. These two protocols provide new, environment-friendly and simple strategies to construct 2,3,5-trisubstituted thiophenes and 3-thienyl disulfides via the formation of two and six C–S bonds, respectively.


 Please wait while we load your content...
Please wait while we load your content...