Facile difluoromethylation of aliphatic alcohols with an S-(difluoro-methyl)sulfonium salt: reaction, scope and mechanistic study†
Abstract
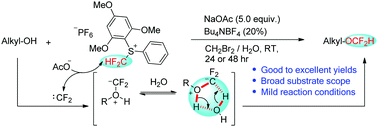
A facile and practical approach for the difluoromethylation of aliphatic alcohols with an S-(difluoromethyl)sulfonium salt was developed. A wide variety of alcohols with broad functional groups are compatible to furnish the corresponding alkyl difluoromethyl ethers in good to excellent yields under mild reaction conditions. Control experiments and DFT computational studies suggest that the difluoromethylation of alcohols mainly proceeds via a difluorocarbene pathway involving a five-membered transition state with the participation of water, whose crucial role in this reaction was also elucidated by control experiments.


 Please wait while we load your content...
Please wait while we load your content...