Lewis acid catalyzed annulation of spirocyclic donor–acceptor cyclopropanes with exo-heterocyclic olefins: access to highly functionalized bis-spirocyclopentane oxindole frameworks†
Abstract
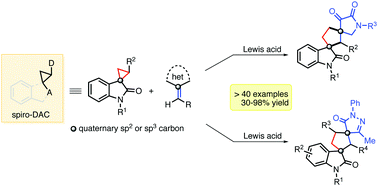
Lewis acid catalyzed highly efficient [3+2] annulation of spirocyclic Donor–Acceptor cyclopropanes (DACs) with exo-heterocyclic olefins is reported to furnish various biologically relevant dispiro-2,3-dioxopyrrolidine[cyclopentane]oxindole and dispiropyrazolone[cyclopentane]oxindole frameworks. This report highlights the use of oxindole-activated spiro-DACs as potential synthons to access complex dispirocarbocyclic oxindoles via ring-enlargement of the former, with high yields and diastereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...