One-pot synthesis of trifluoromethyl amines and perfluoroalkyl amines with CF3SO2Na and RfSO2Na†
Abstract
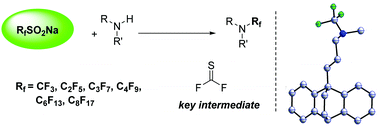
A newly developed CF3SO2Na-based trifluoromethylation of secondary amines has been disclosed, and the method has been successfully extended to the configuration of perfluoroalkyl amines using RfSO2Na, complementing the established synthesis strategy of trifluoromethyl amines. Advantages of the method include good functional group tolerance, mild conditions, and inexpensive or easy-to-handle materials. Mechanistic probes indicate that the thiocarbonyl fluoride formed in situ is the key intermediate in the reaction.


 Please wait while we load your content...
Please wait while we load your content...