Fluorine-substituted quinoidal thiophene with a F–H hydrogen bond locked conformation for high-performance n-channel organic transistors†
Abstract
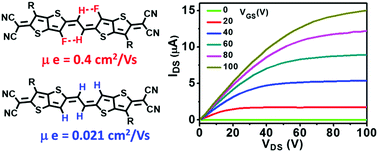
The novel dicyanomethylene-substituted 1,2-bis(thieno[3,2-b]thiophene-2-yl)ethene based quinoidal compounds QBTTE and F2-QBTTE are reported. The F⋯H (vinylene) hydrogen bonds lock the backbone conformation and greatly improve the transistor performance of F2-QBTTE.


 Please wait while we load your content...
Please wait while we load your content...