BiCl3-Mediated direct functionalization of unsaturated C–C bonds with an electrophilic SCF2PO(OEt)2 reagent†
Abstract
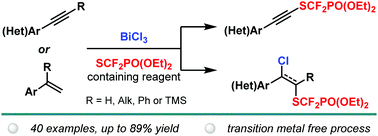
A transition metal-free approach was developed for the direct difunctionalization of disubstituted alkynes and terminal alkenes with concomitant formation of C–SCF2PO(OEt)2 and C–Cl bonds. The BiCl3-mediated reaction offered access to high value-added functionalized scaffolds in a single operation under mild conditions. Extension to SCF2PO(OEt)2-containing alkynes was also studied.
- This article is part of the themed collection: 2019 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...